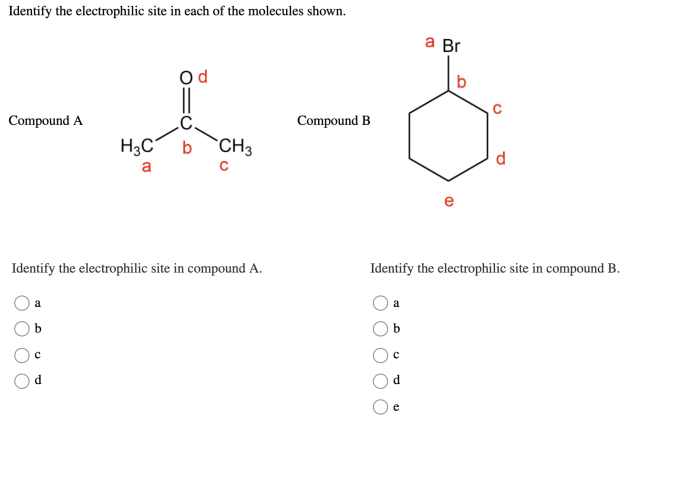
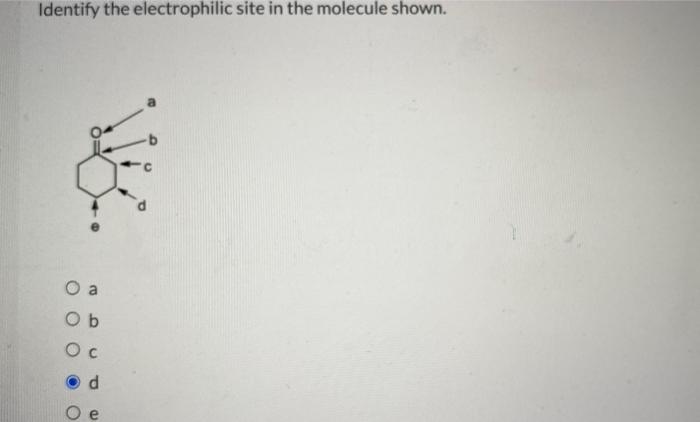
Identify the electrophilic site in each of the molecules shown – Electrophilic site identification, the process of locating reactive sites within molecules, is a fundamental concept in chemistry. Understanding these sites is crucial for predicting and controlling chemical reactions, with applications in diverse fields such as drug design, catalysis, and materials science.
This guide provides a comprehensive overview of electrophilic site identification, covering its definition, characteristics, methods, and applications. We will explore the factors influencing electrophilic site reactivity and discuss how this knowledge can be harnessed to optimize chemical processes.
Electrophilic Site Identification: Identify The Electrophilic Site In Each Of The Molecules Shown
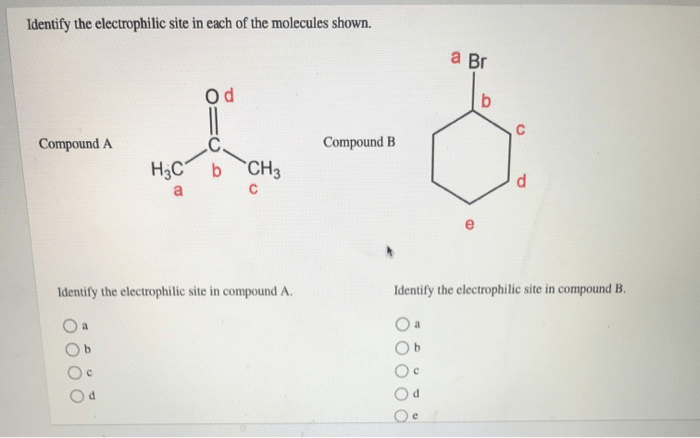
Electrophilic sites are atoms or functional groups in a molecule that can attract electrons from a nucleophile. They are typically electron-deficient and have a positive charge or a partial positive charge.
The electrophilic character of a site is determined by its electronic structure and the surrounding environment. Factors such as the electronegativity of the atom, the presence of electron-withdrawing groups, and the steric hindrance around the site can all affect its electrophilicity.
Electrophilic sites are important in many chemical reactions, including nucleophilic substitution, addition, and elimination reactions. They are also involved in biological processes such as enzyme catalysis and protein-ligand interactions.
Methods for Electrophilic Site Identification
There are a number of methods that can be used to identify electrophilic sites in molecules.
- Molecular orbital theory: This method uses molecular orbital theory to calculate the electron density of the molecule. The electrophilic sites are the atoms or functional groups with the lowest electron density.
- Chemical reactivity indices: These indices are based on the chemical reactivity of the molecule. The electrophilic sites are the atoms or functional groups with the highest chemical reactivity indices.
- Experimental methods: These methods involve performing experiments to measure the reactivity of the molecule. The electrophilic sites are the atoms or functional groups that react most readily with nucleophiles.
Factors Influencing Electrophilic Site Reactivity
The reactivity of an electrophilic site is influenced by a number of factors, including:
- The electronegativity of the atom: The more electronegative the atom, the less electrophilic it will be.
- The presence of electron-withdrawing groups: Electron-withdrawing groups decrease the electron density of the atom, making it more electrophilic.
- The steric hindrance around the site: Steric hindrance can make it difficult for nucleophiles to reach the electrophilic site, decreasing its reactivity.
Applications of Electrophilic Site Identification, Identify the electrophilic site in each of the molecules shown
Electrophilic site identification is used in a variety of applications, including:
- Drug design: Electrophilic site identification can be used to design drugs that target specific proteins or enzymes.
- Catalysis: Electrophilic site identification can be used to design catalysts that can accelerate specific chemical reactions.
- Materials science: Electrophilic site identification can be used to design materials with specific properties, such as conductivity or strength.
Answers to Common Questions
What are electrophilic sites?
Electrophilic sites are regions within molecules that are electron-deficient and therefore attract electron-rich species.
How can I identify electrophilic sites?
Electrophilic sites can be identified using various methods, including resonance structures, molecular orbital theory, and chemical reactivity data.
What factors influence the reactivity of electrophilic sites?
The reactivity of electrophilic sites is influenced by factors such as electronegativity, resonance, and steric effects.